Abstract
Introduction: In clinical trials, and in the real-world, patients with multiple myeloma (MM) discontinue treatment for a variety of reasons including death, disease progression, and toxicity of treatments. Patients may also discontinue trial treatment due to the other reasons such as a physician withdrawing the patient from the trial, or patient deciding to withdraw. The proportion of patients discontinuing treatment for each of these reasons has not previously been reported for MM randomized controlled trials (RCTs). Furthermore, a large number of patients withdrawing from one arm of a trial may raise concern for informative censoring and biased data. To date, there is no prior study evaluating MM RCTs analyzing trial discontinuation and attrition. Due to this, we aimed to assess reasons for discontinuing treatment amongst participants in trials, imbalances in attrition between trial arms, and the practice of reporting this data through a systematic review of all MM RCTs published between 2015-2020.
Methods: Three databases were searched (MEDLINE/PubMed, Embase, and Cochrane Registry of Controlled Trials) for all RCTs in MM from 2015 to 2020. Studies reported as abstracts (without full manuscript available for review), or those that assessed purely supportive care strategies were excluded. We collected documentation of patient discontinuation and reasons for discontinuation in intervention arms, control arms, and in total. As discontinuation due to progression, toxicity and death is expected, we aimed to quantify and assess for rates of discontinuation due to other reasons beside these, such as patient withdrawal or physician decision to withdraw patient. Trials in which the absolute difference in discontinuation rate for these "other” reasons was greater than 5% between the intervention and control arms were thus determined to be outliers. Trials were also assessed for concordance to recommendations outlined by CONSORT guidelines for RCTs.
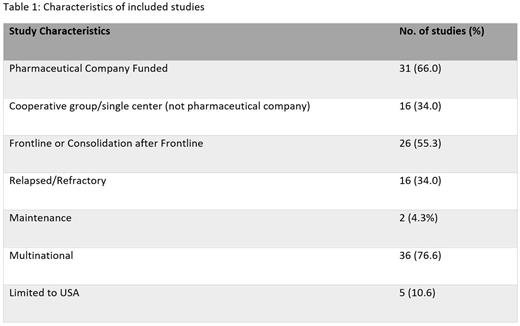
Results: A total 47 RCTs were analyzed that met our inclusion criteria. Table 1 lists characteristics of included studies. Primary endpoint was progression free survival in 33 (70.2%) of these studies and overall survival in 4 (8.5%) studies. In these RCTs, a total of 21,985 patients were randomized to treatment. A total of 10,572 (48.1%) patients had discontinued the RCT by time of primary endpoint ascertainment. The causes of discontinuation in decreasing order of frequency included disease progression (n=4,893; average rate per study (ARPS)=26.4%); toxicity (n=2,691; ARPS=11.7%); patient or physician withdrawal in absence of toxicity/progression/death (n=1,136; ARPS=7.3%) and death (n=527; ARPS=2.6%). A total of 21,540 (98.4%) patients were included in primary endpoint analyses with 45 of 47 trials citing the use of intention to treat analysis. In regard to withdrawal due to reasons other than toxicity, progression or death, a total of 13 (27.7%) of the RCTs were outliers as defined in methods. Amongst these 13 RCTs, attrition was higher in control arm in 10 trials, and higher in intervention arm in 3 trials. Outliers were more commonly found in trials that met their primary endpoint compared to those that did not (10 vs 3 trials, respectively). In the 10 trials meeting their primary endpoint, attrition rate was higher in 8 trials in control arm, and 2 in intervention arm. Although a minority [19 (40.4%)] of trials followed the full recommended guidelines for RCT reporting, the majority 45 (95.7%) of the trials included a CONSORT diagram.
Conclusion: Our analysis demonstrates that although disease progression is the most common reason for discontinuation of treatment in patients with MM on RCT's, over 10% of patients discontinue treatment due to toxicity prior to disease progression or death. Furthermore, 27.7% of trials showed substantial imbalances between the control and intervention arms in the number of patients withdrawn for reasons other than progression, toxicity, or death. This raises concern for informative censoring and bias in results and emphasizes the importance of detailed characterization of reasons for withdrawal in MM RCTs.
Disclosures
Meirson:Purple Biotech: Consultancy. Chakraborty:Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Adaptive Biotech: Consultancy, Honoraria. Sborov:BMS: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bioline: Consultancy; Amgen: Consultancy; Pfizer: Consultancy; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy; Abbvie: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.